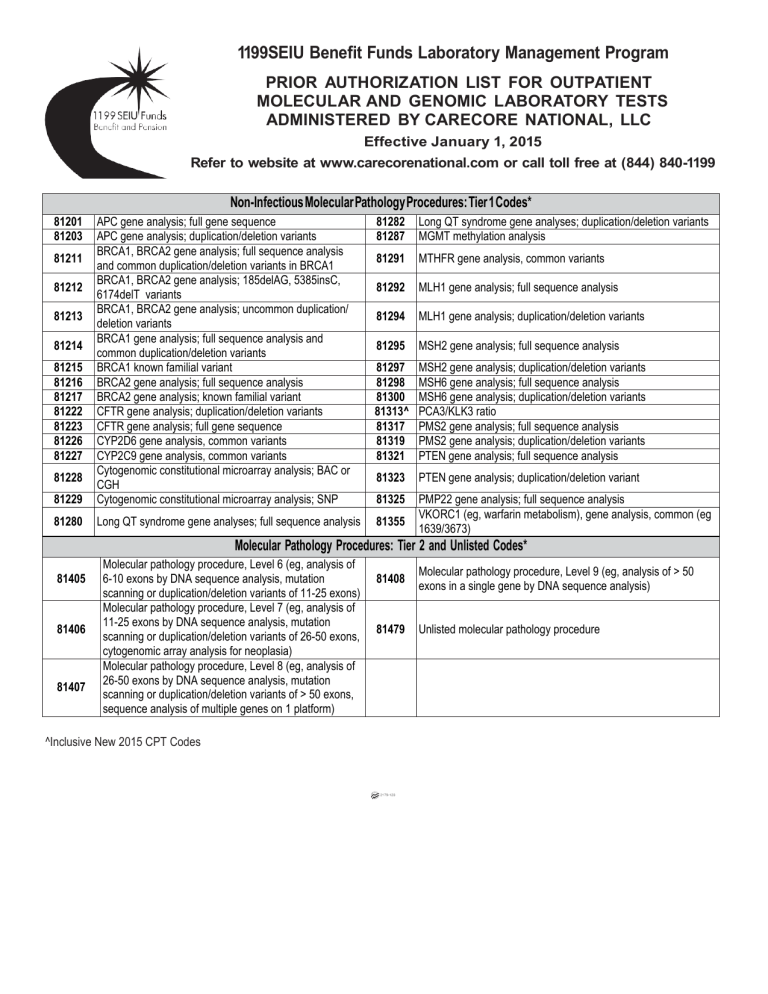
1199SEIU Benefit Funds Laboratory Management Program
PRIOR AUTHORIZATION LIST FOR OUTPATIENT
MOLECULAR AND GENOMIC LABORATORY TESTS
ADMINISTERED BY CARECORE NATIONAL, LLC
Effective January 1, 2015
Refer to website at www.carecorenational.com or call toll free at (844) 840-1199
Non-Infectious Molecular Pathology Procedures: Tier 1 Codes*
81201
81203
81229
APC gene analysis; full gene sequence
APC gene analysis; duplication/deletion variants
BRCA1, BRCA2 gene analysis; full sequence analysis
and common duplication/deletion variants in BRCA1
BRCA1, BRCA2 gene analysis; 185delAG, 5385insC,
6174delT variants
BRCA1, BRCA2 gene analysis; uncommon duplication/
deletion variants
BRCA1 gene analysis; full sequence analysis and
common duplication/deletion variants
BRCA1 known familial variant
BRCA2 gene analysis; full sequence analysis
BRCA2 gene analysis; known familial variant
CFTR gene analysis; duplication/deletion variants
CFTR gene analysis; full gene sequence
CYP2D6 gene analysis, common variants
CYP2C9 gene analysis, common variants
Cytogenomic constitutional microarray analysis; BAC or
CGH
Cytogenomic constitutional microarray analysis; SNP
81280
Long QT syndrome gene analyses; full sequence analysis
81211
81212
81213
81214
81215
81216
81217
81222
81223
81226
81227
81228
81282
81287
Long QT syndrome gene analyses; duplication/deletion variants
MGMT methylation analysis
81291
MTHFR gene analysis, common variants
81292
MLH1 gene analysis; full sequence analysis
81294
MLH1 gene analysis; duplication/deletion variants
81295
MSH2 gene analysis; full sequence analysis
81297
81298
81300
81313^
81317
81319
81321
MSH2 gene analysis; duplication/deletion variants
MSH6 gene analysis; full sequence analysis
MSH6 gene analysis; duplication/deletion variants
PCA3/KLK3 ratio
PMS2 gene analysis; full sequence analysis
PMS2 gene analysis; duplication/deletion variants
PTEN gene analysis; full sequence analysis
81323
PTEN gene analysis; duplication/deletion variant
81325
PMP22 gene analysis; full sequence analysis
VKORC1 (eg, warfarin metabolism), gene analysis, common (eg
1639/3673)
81355
Molecular Pathology Procedures: Tier 2 and Unlisted Codes*
81405
81406
81407
Molecular pathology procedure, Level 6 (eg, analysis of
6-10 exons by DNA sequence analysis, mutation
scanning or duplication/deletion variants of 11-25 exons)
Molecular pathology procedure, Level 7 (eg, analysis of
11-25 exons by DNA sequence analysis, mutation
scanning or duplication/deletion variants of 26-50 exons,
cytogenomic array analysis for neoplasia)
Molecular pathology procedure, Level 8 (eg, analysis of
26-50 exons by DNA sequence analysis, mutation
scanning or duplication/deletion variants of > 50 exons,
sequence analysis of multiple genes on 1 platform)
^Inclusive New 2015 CPT Codes
81408
Molecular pathology procedure, Level 9 (eg, analysis of > 50
exons in a single gene by DNA sequence analysis)
81479
Unlisted molecular pathology procedure
Genomic Sequencing Procedures*
81410^
81411^
81415^
81416^
Aortic dysfunction or dilation; genomic sequence analysis
panel, must include sequencing of at least 9 genes
Aortic dysfunction or dilation; duplication/deletion analysis
panel
Exome sequence analysis
Exome; sequence analysis, each comparator exome
81436^
81440^
81445^
81417^
Exome; re-evaluation of previously obtained exome
sequence
81450^
81420^
Fetal chromosomal aneuploidy genomic sequence analysis
panel, circulating cell-free fetal DNA in maternal blood
81455^
81425^
Genome; sequence analysis
81426^
Genome; sequence analysis, each comparator genome
81427^
Genome; re-evaluation of previously obtained genome
sequence
81465^
81430^
Hearing loss; genomic sequence analysis panel, must
include sequencing of at least 60 genes
81470^
Hearing loss; duplication/deletion analysis panel
Hereditary colon cancer syndromes; genomic sequence
analysis panel, must include analysis of at least 7 genes
81471^
81431^
81435^
81460^
Hereditary colon cancer syndromes; duplication/deletion
gene analysis panel
Nuclear encoded mitochondrial genes, genomic sequence
panel, include analysis of at least 100 genes
Targeted genomic sequence analysis panel, solid organ
neoplasm, DNA analysis, 5-50 genes
Targeted genomic sequence analysis panel,
hematolymphoid neoplasm or disorder, DNA and RNA
analysis when performed, 5-50 genes
Targeted genomic sequence analysis panel, solid organ or
hematolymphoid neoplasm, DNA and RNA analysis when
performed, 51 or greater genes
Whole mitochondrial genome, genomic sequence, must
include sequence analysis of entire mitochondrial genome
with heteroplasmy detection
Whole mitochondrial genome large deletion analysis
panel, including heteroplasmy detection, if performed
X-linked intellectual disability (XLID); genomic sequence
analysis panel, must include sequencing of at least 60
genes
X-linked intellectual disability (XLID); duplication/deletion
gene analysis, must include analysis of at least 60 genes
Multianalyte Assay with Algorithmic Analyses (MAAA)*
81504
Oncology (tissue of origin), microarray gene expression
profiling of > 2000 genes, utilizing formalin-fixed paraffin
embedded tissue, algorithm reported as tissue similarity scores
81507
Fetal aneuploidy (trisomy 21, 18, and 13) DNA sequence
analysis of selected regions using maternal plasma, algorithm
reported as a risk score for each trisomy
Oncology (breast), mRNA, gene expression profiling by real81519^ time RT-PCR of 21 genes, utilizing formalin-fixed paraffin
embedded tissue, algorithm reported as recurrence score
81599
Unlisted multianalyte assay with algorithmic analysis
0004M
Scoliosis, DNA analysis of 53 SNPs, using saliva, prognostic
algorithm reported as a risk score
Oncology (hepatic), mRNA expression levels of 161
genes, utilizing fresh hepatocellular carcinoma tumor
0006M^
tissue, with alpha-fetoprotein level, algorithm
reported as a risk classifier [HeproDX™, GoPath Lab]
Oncology (gastrointestinal neuroendocrine tumors),
real-time PCR expression analysis of 51 genes,
0007M^
utilizing whole peripheral blood, algorithm reported
as a nomogram of tumor disease index [NETest (Wren Lab)]
Oncology (breast), mRNA analysis of 58 genes using
hybrid capture, on formalin-fixed paraffin-embedded
0008M^ (FFPE) tissue, prognostic algorithm reported as a risk
score [Prosigna Breast Cancer Assay (NanoString
Technologies)]
Molecular Laboratory Procedures with HCPCS Codes*
S3800
Genetic testing for amyotrophic lateral sclerosis (als)
S3841
Dna analysis for germline mutations of the ret proto-oncogene
for susceptibility to multiple endocrine neoplasia type 2
Genetic testing for retinoblastoma
S3842
Genetic testing for von hippel-lindau disease
S3845
S3846
Genetic testing for alpha-thalassemia
Genetic testing for hemoglobin E beta-thalassemia
Dna analysis for apoe epsilon 4 allele for susceptibility to
Alzheimer’s disease
Genetic testing for detection of mutations in the presenilin - 1
gene
S3840
S3852
S3855
S3861
S3865
S3866
Genetic testing, sodium channel, voltage-gated, type v,
alpha subunit (scn5a) and variants for suspected brugada
syndrome
Comprehensive gene sequence analysis for hypertrophic
cardiomyopathy
Genetic analysis for a specific gene mutation for
hypertrophic cardiomyopathy in an individual with a known
hcm mutation in the family
S3870
CGH test developmental delay
S3890
Dna analysis, fecal, for colorectal cancer screening
*CPT is a registered trademark of the American Medical Association. Narrative is short description of code.
^Inclusive New 2015 CPT Codes
